Abstract
Background: Pyruvate kinase red cell isoform (PKR) catalyzes the final step of adenosine triphosphate (ATP) production via glycolysis in red blood cells (RBCs), which is critical for maintaining RBC health. PKR activation leading to reduced levels of the glycolytic metabolite 2,3-diphosphoglycerate (2,3-DPG) and enhanced ATP production is under investigation as a potential therapeutic approach in various hemolytic anemias.
AG-946 is a potent, investigational, oral, small molecule activator of wild-type and mutant PKR isoforms. AG-946 has a pharmacokinetic (PK) profile that supports once daily (QD) dosing and has a long duration of pharmacodynamic (PD) effects in normal healthy volunteers (NHVs). Here, we report safety, PK and PD results from the NHV study, and the maximum therapeutic dose (MTD) of AG-946 (NCT04536792).
Methods: In this phase 1, randomized, double-blind, placebo-controlled study, healthy men and women (18-55 years of age) were administered single ascending oral doses (SAD) or multiple ascending oral doses (MAD) of AG-946 or placebo to assess the safety, and PK and PD of AG-946. In SAD, 6 cohorts of 8 subjects each were randomized to receive a single dose of AG-946 (n=6) or placebo (n=2) under fasted conditions, with 2 subjects initially randomized (1:1) and then 6 subjects randomized (5:1) to receive AG-946 or placebo. In MAD, 5 cohorts of 8 subjects each were randomized (3:1) to receive AG-946 or placebo QD under fasted conditions for 14 days. The dose levels studied in SAD were 1, 3, 10, 30, 60, and 100 mg and in MAD were 1, 2, 5, 10, and 20 mg QD. Safety assessments included vital signs, physical exams, electrocardiograms, safety laboratory tests, and adverse events (AEs).
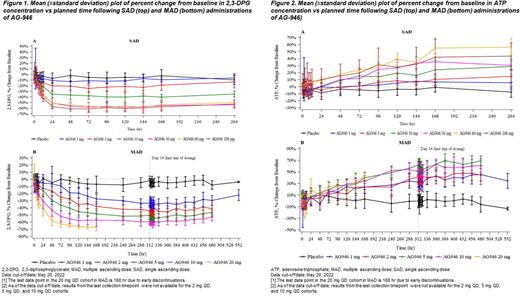
Results: Dose-normalized AG-946 exposures (AUC and Cmax [area under the curve and maximum concentration observed]) increased with increasing AG-946 doses, with an over-proportional increase in exposure over the tested dose range in SAD, and a nearly dose-proportional increase in exposure over the tested dose range in MAD. Dose-dependent and prolonged PD effects (decreases in 2,3-DPG concentrations and increases in ATP concentrations) were observed in both SAD (Figure 1) and MAD (Figure 2). The MTD has been established at a pharmacologically active dose of 5 mg QD.
As of May 2022, 55 subjects in SAD (median age 33 years [range, 21, 55]; 67% white, 33% black) and 40 subjects in MAD (median age 36 years [range, 21, 56]; 48% white, 45% black, 7% other) were randomized to receive AG-946 or placebo. Fifty subjects in SAD and 38 subjects in MAD completed the study. In SAD, 8/55 (14.5%) of subjects experienced ≥1 treatment-emergent adverse event (TEAE). There were no serious AEs (SAEs) or Grade ≥3 AEs. No events were assessed as treatment related by the investigator in SAD.
In MAD, 16/40 (40%) of subjects experienced ≥1 TEAE. There was 1 SAE (Grade 2) of rhabdomyolysis reported in the 2 mg QD cohort that was unrelated to study treatment. A total of 6 decreased platelet events (5 Grade 1, 1 Grade 3) were reported in cohorts receiving doses ≥10 mg QD ([n=1] 10 mg QD; [n=5] 20 mg QD) that were assessed as treatment related by the investigator. The Grade 3 event occurred in the 20 mg QD cohort. No AEs of platelet decrease were observed in other QD cohorts. All 6 decreased platelet events were asymptomatic and reversible with treatment discontinuation. All other AEs reported were nonserious, unrelated to study treatment, and less than Grade 3.
Conclusions: MTD was established in this ph 1 study; AG-946 continues to demonstrate a favorable safety profile, with a PK profile that supports a QD regimen and potent, sustained activation of the glycolytic pathway in RBCs with prolonged effects on PD (2,3-DPG, ATP), supporting further clinical advancement.
Disclosures
Dai Gurov:Agios: Current Employment, Current holder of stock options in a privately-held company. Iyer:Agios: Current Employment, Current equity holder in private company. Claeys:Agios: Current Employment, Current holder of stock options in a privately-held company. Patil:Agios: Current Employment, Current holder of stock options in a privately-held company. Urbstonaitis:Agios: Current Employment, Current holder of stock options in a privately-held company. Xiao:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Callaghan:Agios: Current Employment, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal